Antibody by itself is rather ineffectual in eliminating foreign organisms. However, antibody of the IgM and IgG class can activate the Complement (C') system resulting in a stimulation of different effector functions such as phagocytosis and lysis of foreign organisms.
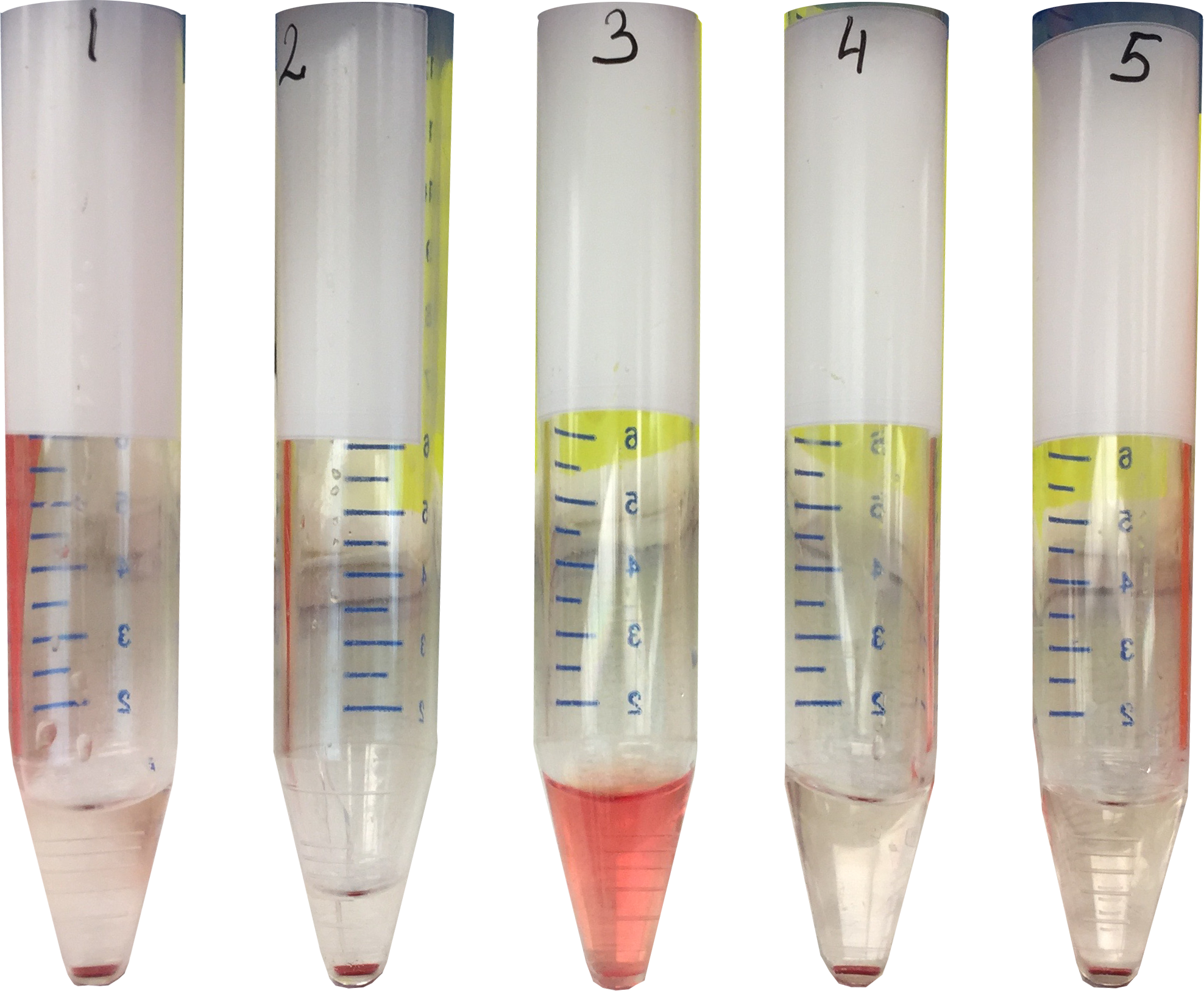
In our experiment Sheep Red Blood Cells (SRBC) are used as a source of antigen to demonstrate the lytic properties of the antigen-antibody-complement complex
In our experiment Sheep Red Blood Cells (SRBC) are used as a source of antigen to demonstrate the lytic properties of the antigen-antibody-complement complex