Research Interests
Biogenesis of Lysosomes
Studies on Lysosomal Prosaposin
Studies on Lysosomal Sphingolipid Activator Proteins (SAPs)
Targeting Mechanisms of Sphingolipid Activator Proteins
Studies on Sortilin
Studies on Reproduction
Techniques Used in Our Laboratory
Biogenesis of Lysosomes
For a long time lysosomes were considered a terminal degradation compartment of the cell. However, this view rapidly changed in the mid 80s by the demonstration that these organelles and their content displayed multiple specialized dynamic functions. From the onset of our research we contributed to this paradigm shift with a series of articles describing the biogenesis and fate of lysosomes in a professional phagocyte, the Sertoli cell. Our kinetic studies demonstrated for the first time that lysosomes turn over with a half-life of approximately 6 hours (Biol. Reprod., 34: 207-218, 1985). We also depicted the precursor compartments of lysosomes using novel markers and we were one of the first laboratories to identify and describe the "endosome" both functionally and morphologically. We used several knockouts (KO) mice models, dominant-negative competitors of sphingolipid activator proteins and lysosomal inhibitors, to demonstrate that multivesicular bodies and lysosomes are transient organelles. We demonstrated that most lysosomal storage disorders occur due to a blockage in the progression of multivesicular bodies to mature lysosomes. We showed that this blockage results in the accumulation of large lysosomes containing extensive amounts of undigested membranes (Biocell 23: 149-160, 1999; Trends in Developmental Biology 2: 13-33, 2007; Histol. Histopathol. 21: 899-913.)
(Top of Page)
Studies on Lysosomal Prosaposin
In the early eighties it was accepted that prosaposin (sulfated glycoprotein-1/SGP-1) was a Sertoli cell secretory protein. In 1987 we demonstrated that in the rat this protein existed in two forms, a secreted isomer of unknown function and a lysosomal isomer, that our and other laboratories, identified as being the homologue of the human lysosomal prosaposin. We demonstrated for the first time by immunogold-labeling the localization of SGP-1/prosaposin within the lysosomes in various cell types including the Sertoli cells. We also cloned the mouse prosaposin cDNA, the mouse prosaposin gene and a variant cDNA of the rat prosaposin. We studied the complete structure of the mouse prosaposin gene and confirmed the existence of an exon also found in the human gene that is spliced in a tissue-specific manner. We pioneered various studies on the effects of prosaposin gene inactivation in various tissues (Mol. Rep. Dev. 48: 1-8, 1997; J. Androl. 21: 765-775, 2000).
(Top of Page)
Studies on Lysosomal Sphingolipid Activator Proteins (SAPs)
Four of the five known sphingolipid activator proteins (SAPs) are small polypetides (10 kDa) termed saposins A, B, C and D. The saposins derive from the partial proteolysis of a common precursor, prosaposin (65 kDa), in the lysosomes. Saposins promote the degradation of glycosphingolipids. Saposins A and C stimulate the hydrolysis glucosylceramide by ß-glucosylceramidase and galactosylceramide, by ß-galactosylceramidase. Saposin B is the activator of arylsulfatase A. Saposin D is thought to activate acid ceramidase. The deficiency of saposin B has been related to a variant form of the lysosomal storage disorder Metachromatic Leukodystrophy (MLS) and the deficiency of saposin C has been linked to a variant form of Gaucher's disease. No deficiency of saposin A or D has yet been reported. However, we have provided unequivocal evidence that the sequence of saposin D is essential for the targeting of prosaposin to the lysosomes (J. Biol. Chem. 275: 24829-39, 2000). Finally, because of the lipid-transfer property of saposins, we have collaborated with Dr. L. Teyton to test the hypothesis that saposins transfer sphingolipids in the endosomes of "antigen presenting cells". Together, we have demonstrated that CD1 molecules required the lipid transfer activity of saposins for lipid loading onto its binding groove (Science 303: 523-27, 2004).
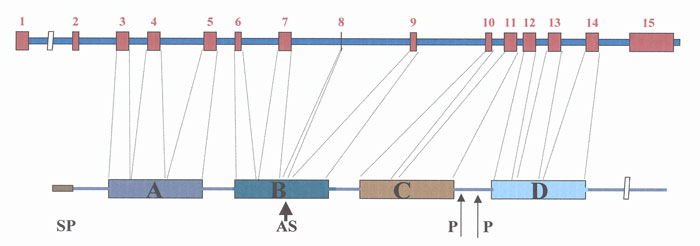
Fig. 1 Mouse prosaposin gene and its translation product prosaposin. Prosaposin contains four saposin domains (A,B,C,D). Exon 8 encodes a QDQ sequence that may or may not be present in domain B due to alternative splicing (AS). Rodents' prosaposins contain a unique proline rich linker region between domains C and D.
(Top of Page)
Targeting Mechanisms of Sphingolipid Activator Proteins
As indicated above, sphingolipid activator proteins (SAPs) are five non-enzymatic cofactors required for the lysosomal degradation of glycosphingolipids. The fifth activator, the GM2 Activator Protein (GM2AP), is the product of a distinct gene and is an essential cofactor of hexosaminidase A in the degradation of GM2 to GM3 ganglioside. Defects in GM2AP are the underlying causes for the AB Variant of GM2 Gangliosidosis characterized by the lysosomal accumulation of undegraded GM2 in neurons, fibroblasts and in several tissues of affected patients. Therefore, the study of this SAP is also relevant to human health. In 1996 we discovered that the lysosomal isomer of prosaposin was sorted in the Golgi apparatus and transported to the lysosomes in a mannose-6-phosphate independent manner. Later we identified the internal amino acid sequence of prosaposin involved in this process and such a study suggested the existence of a lysosomal alternative receptor. Briefly, we performed selective deletions of specific functional domains of prosaposin demonstrating for the first time that elimination of the C-terminus abolished the targeting of prosaposin to the lysosomes (J. Biol. Chem. 275: 24829-39, 2000). When the D domain and the C-terminus of prosaposin were added to the secretory protein albumin, the chimeric protein reached the lysosomes instead of being secreted (J. Biol. Chem. 277: 17188-99, 2002). More recently, we demonstrated that both prosaposin and the GM2AP are targeted to the lysosomes by a similar process. Preliminary evidence from our laboratory also indicated that the binding motif of prosaposin is found in a region of 17 amino acids in the first half of the prosaposin C-terminus (J. Histochem. Cytochem. 58:287-300, 2010). This information is highly relevant since the identified sequence involved in this process may be used for the targeting of proteins and drugs to the lysosomes (lysosomal therapy).
(Top of Page)
Studies on Sortilin
Recently, we have identified a novel lysosomal alternative receptor, sortilin. We have demonstrated that sortilin is involved in the alternative sorting of the SAPs, prosaposin and GM2AP. We and other investigators showed that sortilin has a cytoplasmic GGA (Golgi-localized, γ-ear-containing, ARF-binding protein) binding motif similar to the M6P-R. GGA acts as adaptor proteins bridging the receptor and clathrin, a required step for the targeting of sorted proteins to lysosomes. Interestingly, a dominant-negative GGA construct unable to bind clathrin, prevented the trafficking of prosaposin and GM2AP to the lysosomes. Similarly, we have demonstrated that a dominant negative construct of sortilin lacking the GGA binding motif retained prosaposin and GM2AP in the Golgi apparatus. Although this alternative receptor is implicated in the sorting and trafficking of SAPs, there is no question that sortilin is also involved in the targeting of several other lysosomal hydrolases. With the identification of this novel receptor our laboratory has taken the lead in the study of lysosomal targeting of soluble proteins and our goal is to unfold the important clinical implications of this discovery (EMBO J. 22: 1-8, 2004). Although this alternative receptor was initially implicated in the sorting and trafficking of sphingolipid activator proteins, we have recently found that sortilin is also involved in the targeting of several other lysosomal hydrolases including cathepsin H, cathepsin D (Biochem. Biophys. Res. Comm., 373: 292-297, 2008), and acid sphingomyelinase (Traffic 7: 859-863, 2006). We have cloned the sortilin gene and analyzed its structure (Mol. Rep. Dev. 68: 469-475, 2004) and inactivated this gene by homologous recombination. The inactivation of the sortilin gene decreased but did not abolish the transport of activator proteins to the lysosomes (Exp. Cell Res. 315: 3112-3124, 2009).
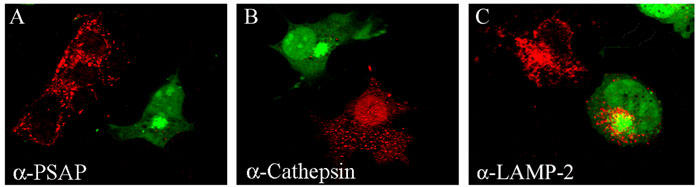
Fig. 2 Transfection of dominant negative GGA lacking the signal to enter clathrin coated cargo vesicles destined to lysosomes. The dominant negative construct was linked to the green fluorescent protein (GGA-GFP), expressed in COS-7 cells and immunostained with anti-prosaposin (A), anti-cathepsin B (B) or anti-LAMP-2 antibodies (C). GGA-GFP transfected cells can be recognized by their green fluorescence. While anti-prosaposin (A) and anti-cathepsin B (B) antibodies produced intense punctate staining in non-transfected cells (red fluorescence), transfected cells (green fluorescence) are not stained by these antibodies. Cathepsin B is targeted to lysosomes via the mannose 6-phosphate receptor (M6P-R), which interacts with GGA. Therefore, the dominant negative GGA abolished the transport of this hydrolase to lysosomes. Prosaposin does not use the M6P-R but requires GGA to be targeted to lysosomes. Sortilin, a transmembrane protein that uses monomeric GGA, was considered the putative receptor for prosaposin. (C) LAMP-2 reached the lysosomes in GGA-GFP transfected cells because this transmembrane protein uses tetrameric adaptor protein-3 (AP-3).
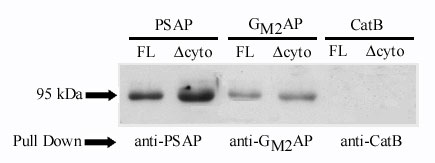
Fig. 3 Co-immunoprecipitation showing association between SAPs and sortilin. Prosaposin, GM2AP or cathepsin B and full length sortilin (FL) or truncated sortilin lacking the cytoplasmic region (Δcyto) were in vitro translated in a reticulocyte lysate system. For visualization, the proteins were labeled using Transcend Non-Radioactive Translation Detection System (Promega). The translated proteins were mixed and incubated with appropriate antibody. The complex was pulled down using protein A beads, suspended in reducing sample buffer, boiled at 100oC for 5 min, run on 12.5% acrylamide gel, and transferred to nitrocellulose paper. The first 2 lanes (left) demonstrates that anti-prosaposin antibody pulls-down FL sortilin (95kDA) and Δcyto sortilin (90kDa). The lanes in middle show that anti- GM2AP antibody also pulls-down FL sortilin (95kDa) and?cyto sortilin (90kDa). On the other hand, the last two lanes (right) show that anti-cathepsin B antibody did not pull down sortilin.
(Top of Page)
Studies on Reproduction
During the past 5 years, in collaboration with Dr. Scott Argraves (Medical University of South Carolina), we have pioneered functional studies on the role of the LDL receptor-like related protein-2 (LRP-2/megalin) in the male reproductive system. We demonstrated for the first time that apoliprotein J is the major ligand of megalin in the epididymis and that both proteins may play a role in sperm maturation and sperm capacitation. In addition, we have identified in the sperm plasma membrane several proteins involved in cholesterol efflux, a process that is essential for fertilization (Biochem. Biophys. Res. Comm. 373: 292-297, 2008).
(Top of Page)
Techniques Used in Our Laboratory
Light (LM) and electron microscope (EM) immunocytochemistry, confocal microscopy, molecular cloning, RNA interference (RNAi), homologous recombination, transgenesis, hybridization techniques, proteomics.
(Top of Page)