Calculations of serial dilutions
Correct pipetting is critical when doing experiments in the Lab, especially when doing serial dilutions, which will be demonstrated in the video below
Serial vs Simple dilution
The dilution process can be a one step process or the steps may be repeated several times. If it is a single-step process it is called a
simple dilution and when the steps are repeated, it is called a
serial dilution.
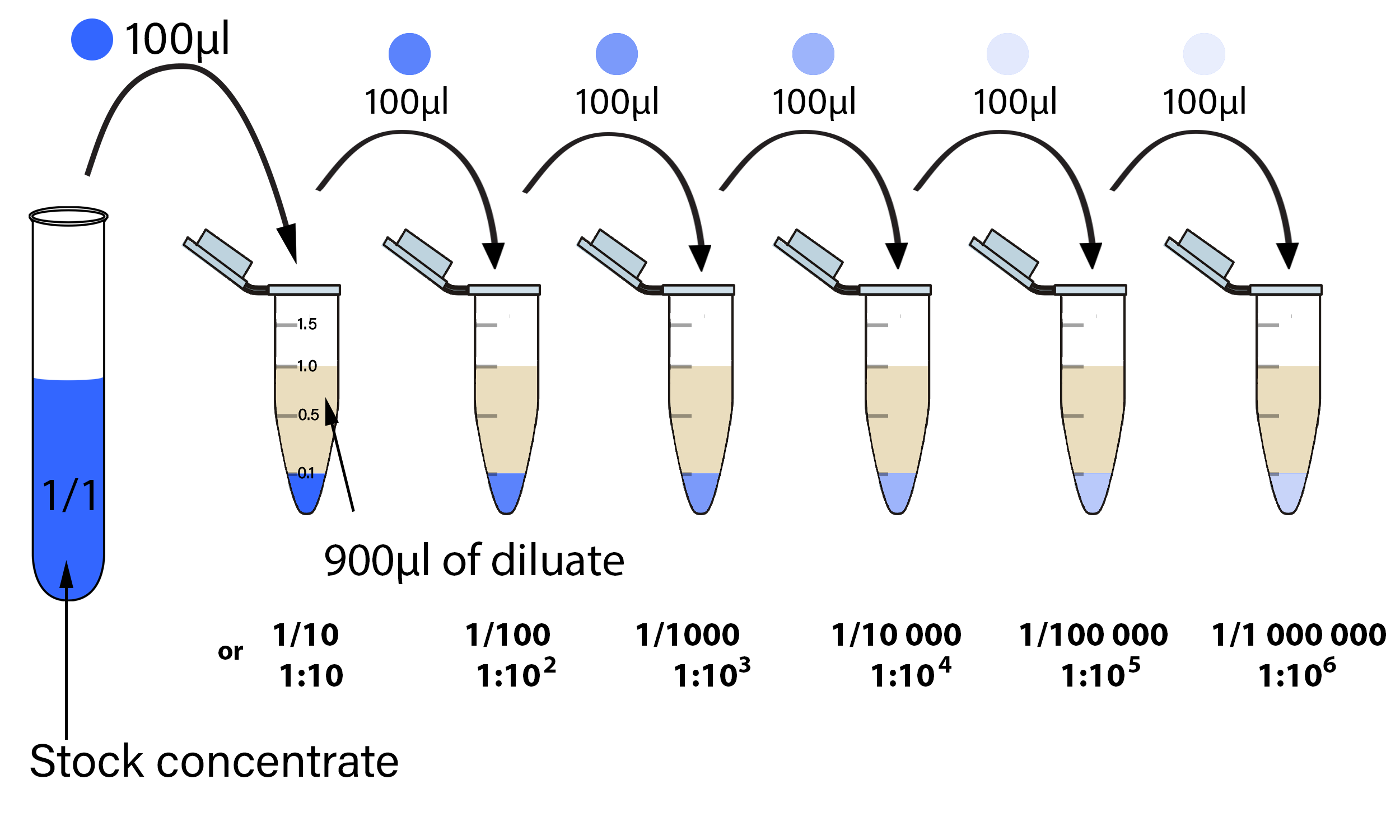
Serial Dilution
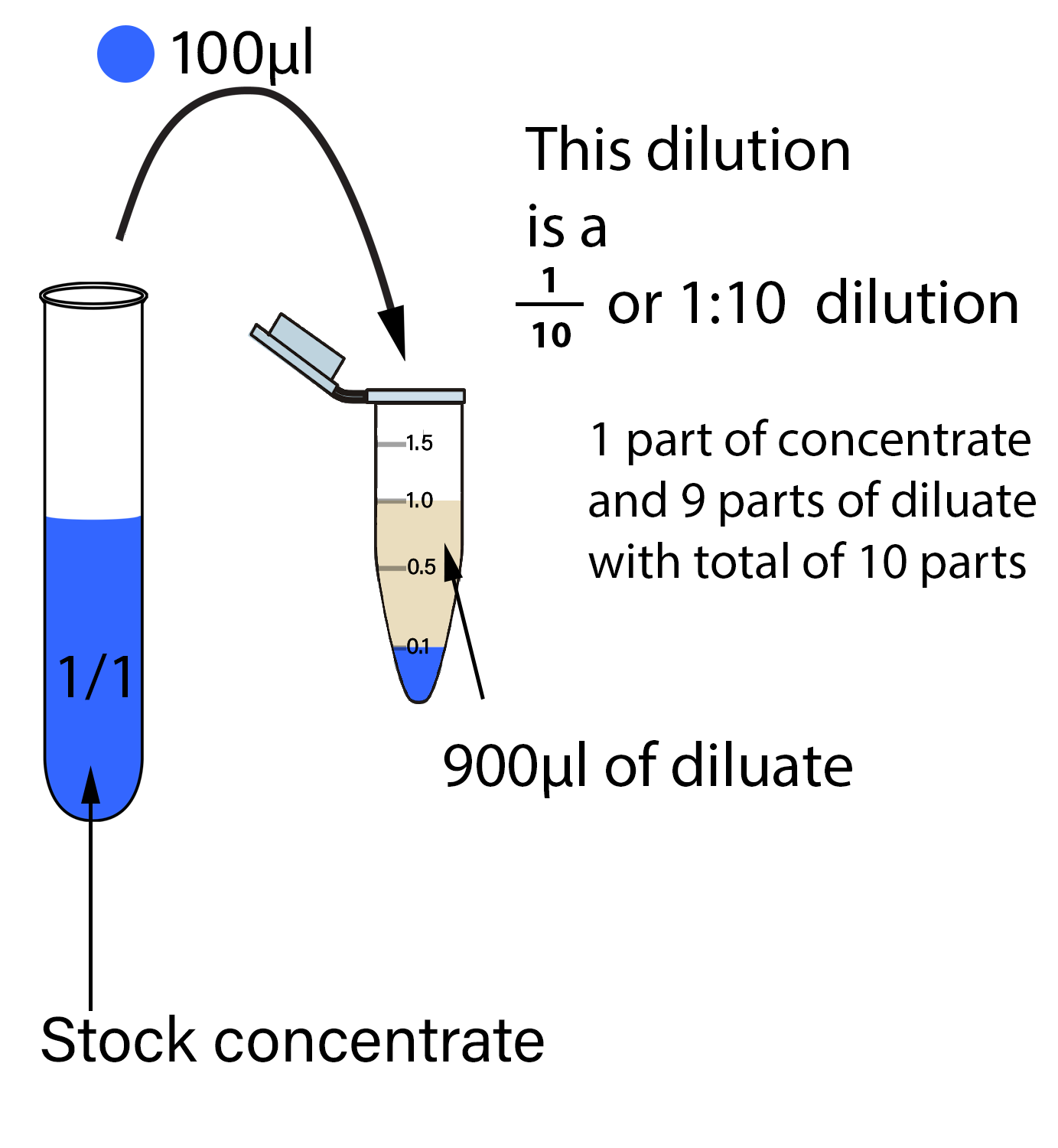
The first step in making a serial dilution is to take a known volume (i.e. 100µl) of stock extract (diluate) and place it into a known volume of diluting solution (diluent) (i.e. 900µl). This produces 10 µl of the dilute solution. This dilute solution has 1 µl of extract /10 µl of total solution, producing a 10-fold dilution. Therefore, the amount of stock in each ml of the diluted solution is 0.1 ml.
This technique used to make a single dilution is repeated sequentially using more and more dilute solutions as the "stock" solution. At each step, 100 µl of the previous dilution is added to 900 µl of diluent. Each step results in a further 10-fold change in the concentration from the previous concentration.
If you want to make a 5-fold, 1/5 or 1:5 serial dilution you simply use 4 times (n-1) the amount of diluent in proportion to amount of your sample so the total volume obtained is 5 times of the original volume.
Simple Dilution
Terminology:
The
diluate, concentrate or solute refers to the sample to be diluted and the
diluent or solvent is the liquid used to dilute it.
The formula defining dilution:
dilution = volume of diluate / total volume
And:
DF = total volume / volume of diluate
Total volume (final volume)= volume of dilute (initial volume) + volume of diluent (solvent)
DF - dilution factor, a dimensionless value (no unit), a factor by which the stock solution is diluted.
- Top -