G protein-coupled signal transduction systems
![]()
Hébert Lab,
Department of Pharmacology and Therapeutics,
McGill University
|
Research in my lab is is centered broadly around the theme of G
protein-coupled signal transduction systems. These signalling
systems are activated by agonists that bind to G protein-coupled
receptors (GPCRs, alternatively we use the term heptahelical
receptor or 7TM-Rs) leading to the regulation of effector
proteins (e.g. enzymes and ion channels) by a transducer. We are
interested in 1) novel signalling complexes and pathways
associated with alternative subcellular localization of GPCRs
and G proteins and how they might be involved in a rare
neurodevelopmental disorder, 2) designing and validating
biosensors to track signalling events and allosteric modulation
of GPCRs. 3) measuring such events in primary cells, inducible
pluripotent stem cells, and in living animals, and 4) basic
mechanisms of how GPCR signalling systems are wired, and the
roles that these architectural features of signalling complex
design might play in cardiovascular and neurodegenerative
diseases. These projects
are currently funded by CIHR, MITACS and the Weston Brain
Institite.
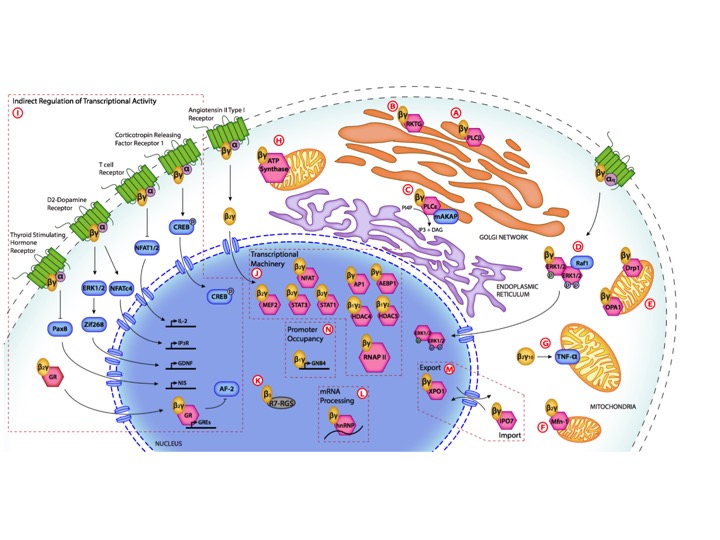
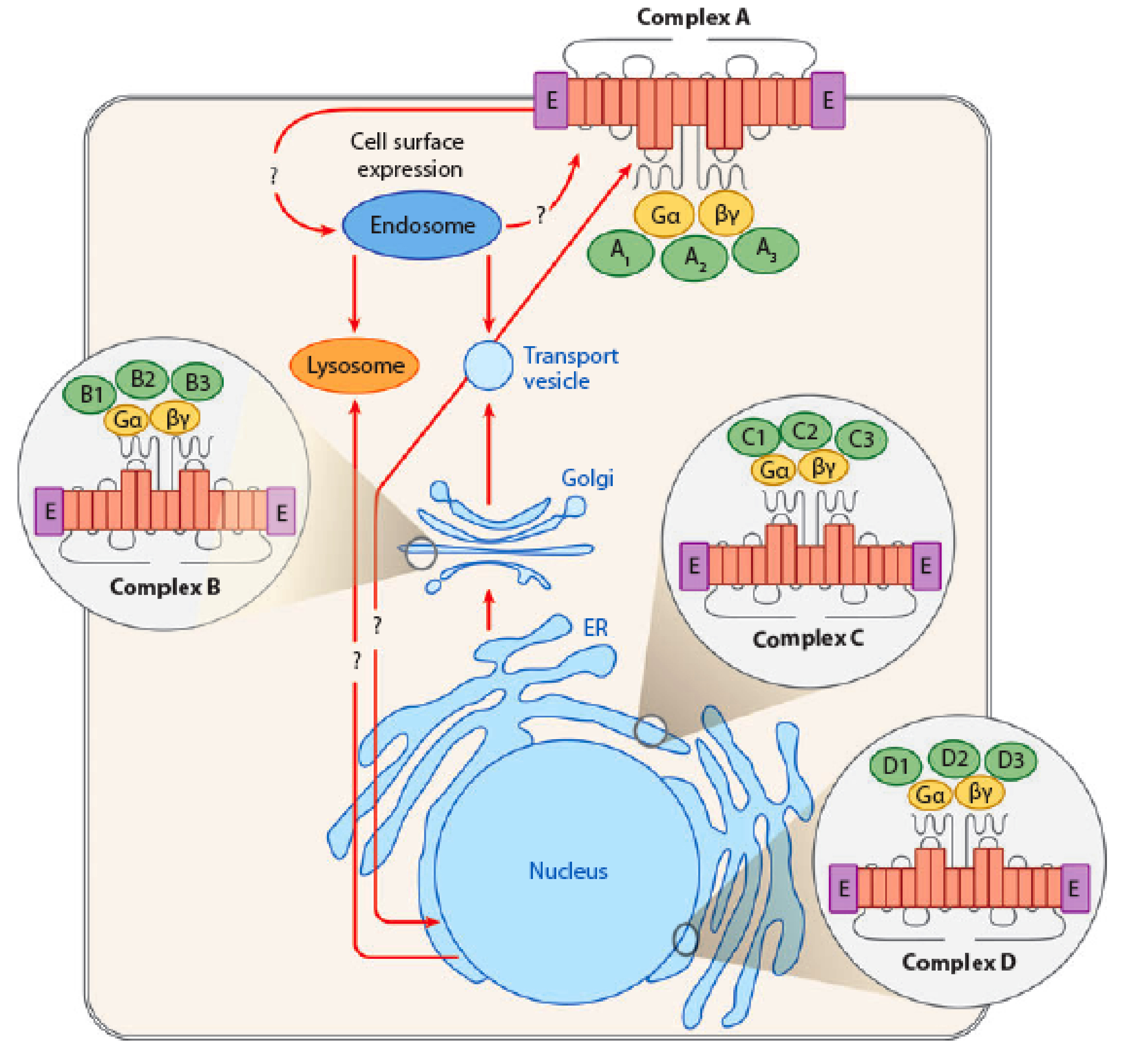
1) Alternate subcellular destinations for 7TM-R signalling complexes. In recent years we have come to realize
that the cell surface is not the only site where 7TM-Rs are
functional. These receptors, and perhaps more interestingly,
fragments derived by regulated proteolytic events as well as
their associated signalling machinery have functions in other
cellular compartments including the ER, Golgi and nuclear
membranes. An increasing number of 7TM-Rs have also been
demonstrated to be targetted to the endomembrane locations as
have their associated signalling molecules. Several lines of
investigation led us to an interest in signalling by internal
GPCRs. More recently we have been studying how G proteins might
be transcriptional regulators during the cardiac fibrotic
response and more generally how such signalling might be
understood and used for drug discovery in cardiovascular disease.
.
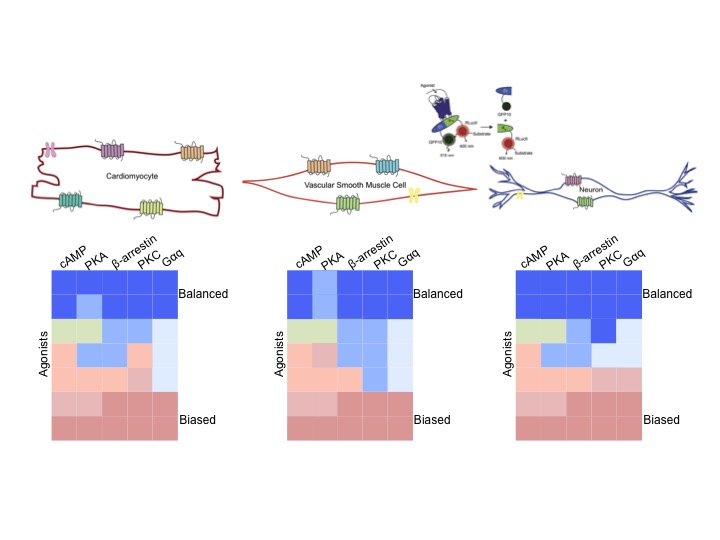
2) Developing biosensors to track GPCR signalling.
Over the past few years, we have helped conceive, design,
produce and validate over BRET-based biosensors to track
receptor signalling by measuring activation of heterotrimeric G
proteins, downstream effectors, production of second messengers
and the recruitment of GPCR-interacting proteins
as well as
conformational biosensors to
how
cellular environment influences readout from different biosensor
platforms, i.e. whether our different platforms are “portable” from cell type to
cell type. The latter set of conformational biosensors do not
require any knowledge of downstream signalling events and will
complement signalling biosensors in this regard as we move
across different cell types.
3) Moving biosensors into mor relevant physiological contexts.
Although technical prowess to screen for drugs has increased
dramatically, translation into the clinic has stagnated. This is
likely due to 1) our rudimentary understanding of disease
mechanisms, and 2) our increasing use of generic, cell-based
screens (using heterologous systems such as HEK 293 cells) which
have moved us away from biologically relevant tissues, organs
and patients.
To optimize integration of
pharmacological screening data and the translational outputs
from such research for both common and rare diseases, we need a
richer and more complete understanding of patients in the real
world.
Our goal here is to develop better preclinical
models linking clinical samples with drug screening technologies
and use patient-derived inducible pluripotent stem cells (iPSCs)
and ultimately organoids, which will feed the disruptive
development of personalized treatment with better outcomes for
patients, through improved understanding of individualized
disease mechanisms and therapeutic responses, new molecular
diagnostics and targeted therapies. We have adopted a
number of approaches including using adeno-associated versions
of our current biosensor panels in patient-derived cells and
also in primary cells isolated from animals and more recently in
the brains of intact behaving animals.
|