|
Blood
Laboratory |
Red cell fragility
>
Osmotic hemolysis |
| |
Cell membranes are semipermeable barriers, and osmotic
gradients are established between intracellular and extracellular fluids which can cause
water to flow into and out of the cells. The amount of osmotic pressure depends upon the
difference between the concentration of non-diffusible ions on each side of the
membrane. |
|
The theoretical background
for this exercise is to be reviewed in your text book. |
|
The
intracellular fluid of erythrocytes is a solution of salts, glucose, protein and
hemoglobin. A 0.9% NaCl solution is said to be
isotonic: when
blood cells reside in such a medium, the intracellular and extracellular fluids are in
osmotic equilibrium across the cell membrane, and there is no net influx or efflux of
water. |
|
When subjected to hypertonic media
(e.g. 1.8% NaCl), the cells lose their normal biconcave shape, undergoing collapse
(leading to crenation) due to the rapid osmotic efflux of water. |
On the other hand, in a hypotonic
environment (e.g. 0.4% NaCl or distilled water), an influx of water occurs: the cells
swell, the integrity of their membranes is disrupted, allowing the escape of their
hemoglobin (hemolysis) which dissolves in the external medium. |
|
 Crenation Crenation
|
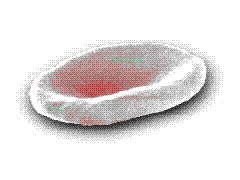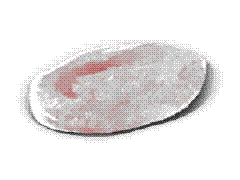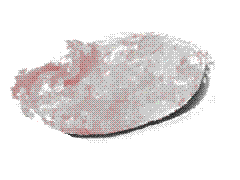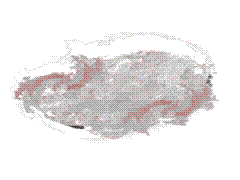 Hemolysis Hemolysis |
|
In this experiment, we make use of the property that the
osmotic fragility (or susceptibility to hemolysis) of erythrocytes is not uniform, and the
number of cells undergoing hemolysis depends on the degree of hypotonicity of the
extracellular medium. The concentration of liberated hemoglobin in each test medium is an
index of the extent of osmotic hemolysis. Your task is to examine the relationship between
extent of hemolysis and osmolarity of the medium in which the erythrocytes are suspended. |
|
To
continue with the procedure of erythrocyte fragility, click here |
| |
|
|
|
|
|
|
| |
| |
| |
| |
|
| |
| |
| |
|
| |
| |
| |