|
RMP
Laboratory |
RMP >
Theory |
| |
All cells under resting conditions have an
electrical potential difference across the plasma membrane such that the
inside of the cell is negatively charged with respect to the outside.
This potential is the resting membrane potential; its
magnitude depends on the type of cell, but usually ranges between -60
and -90 mV. By convention the polarity (positive or negative) of the
membrane potential is stated in terms of the sign of the excess charge
on the inside of the cell |
|
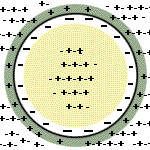
The membrane potential can be accounted for
by the fact that there is a slightly greater number of negative charges
than positive charges inside the cell and a slightly greater number of
positive charges than negative charge outside. The excess negative
charges inside the cell are electrically attracted to the excess
positive charges outside the cell, and vice versa. |
 |
|
Thus, these excess ions collect along a thin
shell on the inner and outer surfaces of the plasma membrane, whereas
the bulk of the intracellular and extracellular fluid is electrically
neutral. The total number of positive and negative charges that have to
be separated across the membrane to account for the potential is an
insignificant fraction of the total number of charges actually in the
cell. |
|
The resting membrane
potential is determined mainly by two factors:
Sodium, potassium, and chloride ions are
present in the highest concentrations and therefore generally play the
most important roles in the generation of the resting membrane
potential. |
|
Ion |
Extracellular
mmol/l |
Intracellular
mmol/l |
| Na+ |
150 |
15 |
| Cl- |
110 |
10 |
| K+ |
5 |
150 |
|
The sodium and chloride ion concentrations
are lower inside the cell than outside, and the potassium concentration
is greater inside the cell. |
|
These concentration differences for sodium
and potassium are due to the action of a membrane active transport
system which pumps sodium out of the cell and potassium into it. |
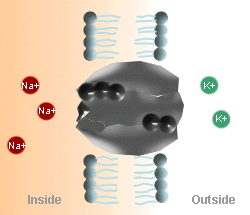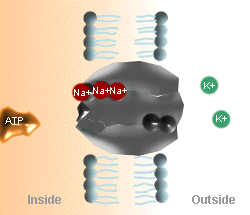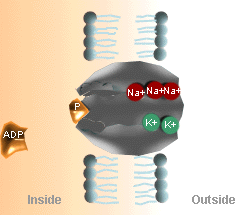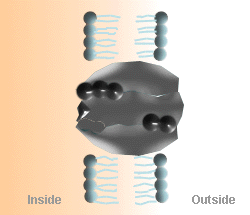
The Na+
- K+ Pump Cycle
|
A. Three Na+ ions on the inside of the cell membrane bind
to the pump protein (carrier molecule).
B. The pump protein is phosphorylated by ATP. |
C. The 3 Na+ ions are released to the outside of
the cell membrane, and the outside K+ binds to the pump protein.
D. K+ is released to the inside of the cell and the
pump protein releases the phosphate and returns to its original
conformation. |
|
 |
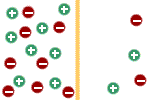 |
To understand how the concentration
differences for sodium and potassium (maintained by the membrane pumps)
create membrane potentials, let us consider the following situation: let
us assume that the membrane is permeable only to potassium but
not to sodium. Therefore, potassium can diffuse through the membrane but
sodium cannot. Initially there is no potential difference across the
membrane because the two solutions are electrically neutral; i.e., they
contain equal numbers of positive and negative ions. |
|
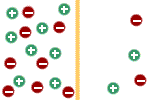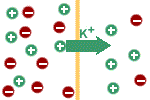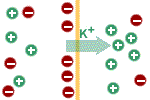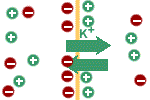
Inside --> Outside |
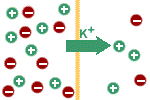 |
Because the membrane is
permeable to potassium ions, they will flow down their concentration
gradient; i.e. towards the outside of the cell. There is also a
concentration gradient favouring sodium diffusion in the opposite
direction but the membrane is not permeable to sodium. Accordingly,
after a few potassium ions have moved out of the cell, the cell will
have an excess of negative charge, whereas the outside solution will
have an excess of positive charge; i.e., a potential difference will
exist across the membrane. |
|
Inside --> Outside |
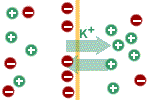 |
The potential difference itself influences
the movement of potassium ions. They (being positive) are attracted by
the negative charge on the intracellular side of the membrane and are
repulsed by the positive charge on the extracellular side of the
membrane. As long as the force due to the concentration gradient driving
potassium ions outside the cell is greater than the electrical force
driving it in the opposite direction there will be net outside movement
of potassium ions; the cell will become more and more negative until the
electric force opposing the exit of potassium ions outside of the cell
equals the force due to the concentration gradient favouring its exit.
|
|
Inside --> Outside |
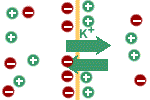 |
The membrane potential at which the
electrical force is equal in magnitude but opposite in direction to the
concentration force is called the equilibrium potential for that ion. At
the equilibrium potential there is no net movement of the ion because
the opposing forces acting on it are exactly balanced. |
|
Inside --> Outside |
|
|
 |
|
|
To continue to the next section: Theory Nernst, click here |