|
Immunology Laboratory |
Plaque Forming Cell
Response
(Not performed during the lab) |
| |
Two of the immune function
assays, relying on antibody response, are the Plaque Forming
Cell Assay (PFC) and the hemagglutination test. The
PFC assay measures IgM producing cells and:
|
|
Background |
|
In the mid sixties, Jerne developed a plaque
assay, based on local hemolysis in gel, to identify and count individual
antibody forming cells. The original technique has been modified in
different laboratories. The technique used in the teaching laboratory
is a modification of the method described by Cunningham and Szenberg
(1968): it is the "direct plaque forming cell (PFC) assay and only
detects IgM secreting cells. An " indirect PFC assay" can be used to
detect IgG secreting cells. |
|
References: Jerne, N.K. and Nordin, A.A. 1963. Science 140:
405.
Cunningham, A.J. and Szenberg, A. 1968. Immunology 14: 599.
Kongshavn, P.A.L. and Lapp, W.S 1972. Immunology 22: 227 |
|
Immunized animal versus non-immunized
animal |
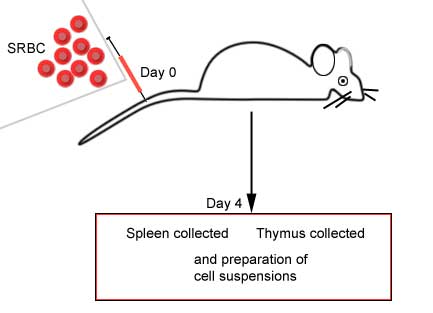 |
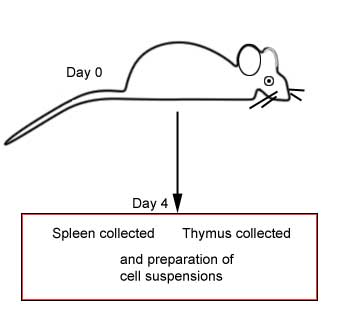 |
|
Procedure |
Preparation of a cell
suspension:
|
Spleen cell suspensions are prepared by
gently tamping the spleen through a 60-mesh stainless steel screen, and
collecting the cells in balanced salt solution (BSS). The spleen cells
are washed and made up to 15 ml with BSS. SRBC are washed twice and made
up to a 10% concentration. Complement (Gibco) is diluted 1/20 with BSS.
All stock solutions are kept on ice water until used. |
|
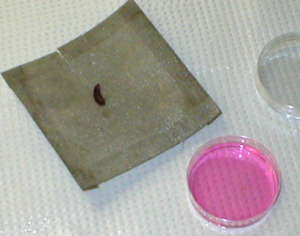
The excised mouse spleen is placed on a
60-mesh stainless steel screen, over the bottom of a small empty Petri
dish. |
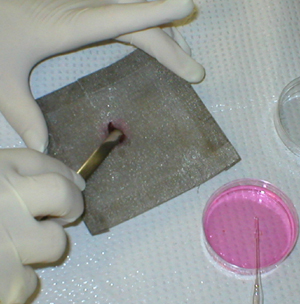
The spleen is gently teased through the mesh
with a spatula |

and rinsed with balanced salt solution. |
 |
 |
|
 |
 |
 |
All the cells are
transferred to a conical test tube and the volume adjusted to 15 ml. |
|
Washing cells:
|
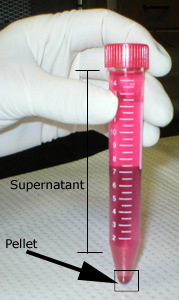
The tube filled with the cell suspension is centrifuged. The
supernatant is aspirated and discarded. The remaining pellet,
constituted of intact spleen cells, is resuspended in fresh
balanced salt solution. The tube is once more centrifuged; the
supernatant is also discarded and the pellet is resuspended in
more balanced salt solution. The cells are washed this way three
times with balanced salt solution. |
|
 |
|
|
Adding cells, SRBC and
Complement to slide chambers (performed in the lab): |
|
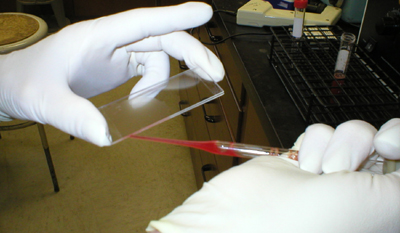
The test consists of mixing
0.05 ml of spleen cells, 0.070 ml of SRBC and 0.5 ml of the complement
solution in a test tube at 37C. The whole mixture is immediately
withdrawn and put into chambers prepared by gluing two 75 x 25 mm slides
together with double-sided tape. Four to five slide chambers are
necessary for each sample, filling up the last chamber if necessary with
a blank solution of SRBC and complement in the same concentrations as
for the test mixture. |
|
|
|
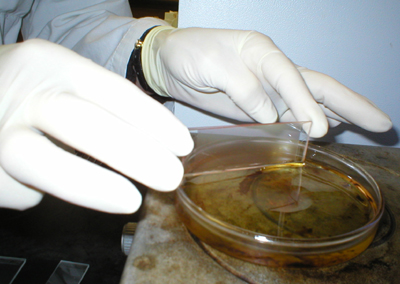
The slide chambers are sealed with paraffin
wax, and incubated at 37C for 45-60 minutes. In earlier experiments, a
30 minute incubation period was used, but this proved insufficient time
for full development of all plaques. |
|
|
 |
The number of PFC are counted by both macro-
and microscopic examination. |
|
Since time does not permit you to make your
own cell suspension, you are provided with spleen cell suspensions as
well as thymus cell suspensions from the two experimental groups.
Remember to always mix the cell suspension as you use it: the cells
settle down at the bottom of the tube rather quickly (you want to make
sure you add a uniform cell suspension into each of the slide chambers). |
|
All other reagents, made up to the proper
concentrations, are labelled and placed at central locations in the
laboratory or at your bench. Each laboratory team should assay 4
different experimental groups of mice. |
|
In brief: |
| Place
into a tube: |
- 50
ml
of a spleen from animal A, or from animal B, or thymus cell from
animal A, or B
|
- 0.50 ml of C' into each tube
|
- 70
ml
of SRBC (1/10 concentration) into each tube
|
| Warm
to 37C in the water bath (30-60 seconds) |
| Use a Pasteur pipette
to transfer the contents of all tubes into the slide chambers
(4-6)). Fill the last chamber with the blank solution (C' + SRBC) if
necessary. |
| Seal
the chambers by dipping both edges into the warm paraffin wax. |
| Place in the incubator
for one hour. |
|
Remove from the incubator and count. |
|
|
Results |
|
You should see on some of your slides clear
areas where RBC lysis has occurred: Antibody-forming cells are measured
by mixing the spleen cells from the immunized animal with the Antigen
(Sheep Red Blood Cells). Following incubation, the red cells surrounding
the cells secreting specific antibody become coated with the antibody
and may be lysed by complement. |
|
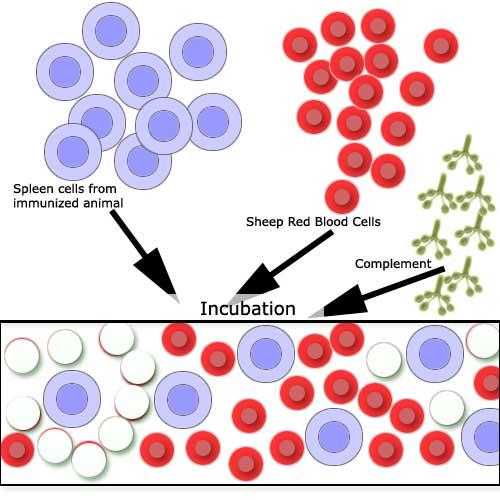
|
|
A slide chamber, showing
clear areas (a light beam was directed onto the slide). |
 |
|
|
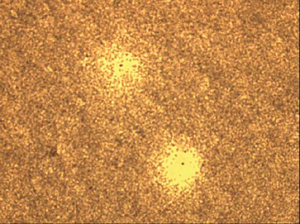 |
View under the microscope: 2 plaque-forming cells. |
|
From your results, identify the two groups
of animals (A or B?) - normal or immunized with SRBC. Assume the
following information for each cell suspension: |
|
Organ |
Total lymphoid cell count |
Total volume of cell suspension |
|
Spleen |
80 x 106 cells |
15 ml |
|
Thymus |
90 x 106 cells |
15 ml |
|
-
Determine the total
number of PFC per organ: there are 80 x 106
lymphocytes of which
60% are B cells and the doubling time is 15- 18 hours.
-
Determine the number of
PFC per 106 lymphoid cells: you are estimating the number
of clones that responded by manufacturing antibody.
-
Obtain results from
other laboratory teams and calculate the: mean, standard deviation and
standard error of the mean
|
|
Points of discussion |
|
• The number of PFC in each organ/106
cells |
|
• The number of PFC in the non-immunized
group |
|
• How do you account for background PFC in
the non-immunized group? |
|
• Immunological specificity |
|
• Organs active in antibody production |
|
• Using the PFC assay how could you
demonstrate that antibody forming cells are not T lymphocytes? |
|
|