|
Compound Action Potential |
Background >
Recording technique |
| |
In this laboratory, you will
be recording an extracellular,
biphasic, compound action potential.
Clarifying those three terms in the context of the experiment
will help you to understand and interpret your results. |
|
|
|
Intracellular versus
Extracellular recording |
|
|
One can measure a single trans-membrane potential by inserting a glass pipette into one cell and recording
the potential changes with respect to an extracellular reference electrode. This
intracellular
technique is used, for example, to record the resting membrane potential
of a muscle in the Resting Membrane Potential Lab.
The intracellular recording technique does
allow for very accurate assessment of the electrical activity of a single cell, but it is
very difficult to do in vertebrate nerve fibres and can involve considerable damage to the
membrane around the electrode tip. |
|
A far less demanding technique,
extracellular
recording, involves placing one electrode in close proximity to the excitable cell and the
reference electrode at some location in the extracellular fluid. This arrangement
records potential changes at the membrane surface rather
than across the membrane. |
|
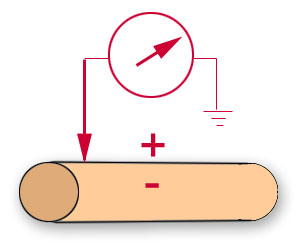
Extracellular positioning of
the recording electrode
|
|
Action potentials recorded extracellularly
differ from those recorded intracellularly in several important respects. The
size
of any one action potential will be obviously reduced. The
shape
of the
waveform for any one action potential will
depend on the exact geometry of its contact
with the electrode. Extracellular techniques are therefore better suited where one only
wants to know that an action potential has occurred or to record the activity of an entire
population of cells. |
|
|
|
|
In this lab you will study the response of the sciatic
nerve of the frog to electrical stimulation using two pairs of
stainless steel wire electrodes that
are both in contact with the nerve, therefore recording the potential difference between
two points on the nerve. There are 12 such recording electrodes in the nerve bath,
but only two can be connected to the differential amplifier at any one time.
|
|
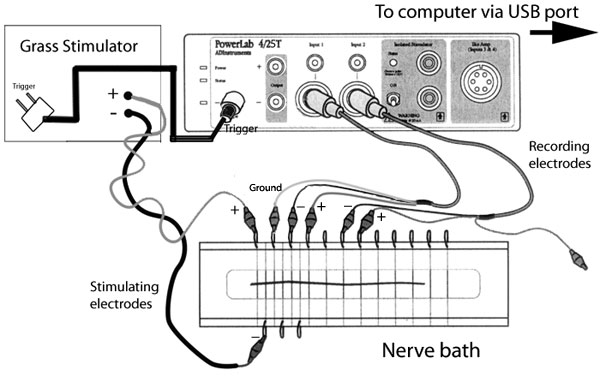
Modified from ADinstruments.
All Rights Reserved. |
|
Delivering a sufficiently large stimulus to
the nerve will result in an action potential that is quite a bit larger than a single
intracellular action potential but looks remarkably similar. |
|
This compound action
potential (CAP) is the algebraic summation of all the action potentials produced by all
the fibres that were fired by that stimulus. The nerve is made of thousands of axons
whose size, myelination and position with respect to the stimulating and recording
electrodes all affect the size of their contribution to the compound action potential. |
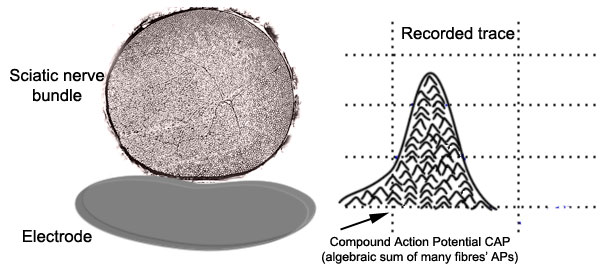 |
|
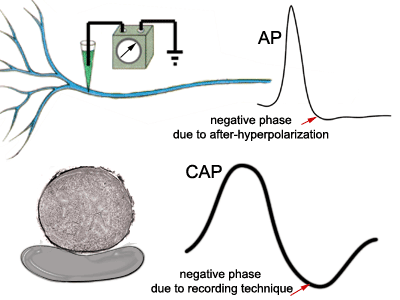
Both the classic intracellular action
potential and the compound action potential are biphasic. In other words, they have
both positive and negative deflections, but for different reasons. The negative
phase of the intracellular action potential is attributed to the mechanism of
after-hyperpolarization. The negative phase of the CAP is due to the manner in which
it is recorded, which will be explained below. |
 |
|
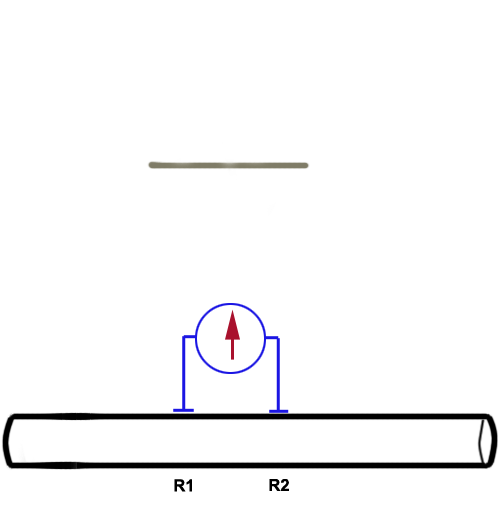
There are two wire recording electrodes (R1
and R2) touching the
nerve, each connected to one input of the differential amplifier. The animation
below illustrates how the shape of the CAP depends on the position of the two
electrodes with respect to the travelling CAP. For an in-depth explanation, please
read on below. |
|

|
Before the stimulus is delivered, both wires should be
measuring basically the same voltage. There will be no deflection recorded because
the amplifier takes the difference of the two inputs before passing the signal on to the
A/D converter.
|
|
|
The situation changes as the CAP travels along the
nerve. The shape of the CAP will depend on the relationship between the
inter-electrode distance, the length of the axon segments depolarized by the action
potentials, and the conduction velocities of the axons.
When the CAP has reached the
first recording electrode (R1, proximal), the proximal electrode
becomes transiently negative to the distal electrode; the potential
difference between the two is detected and the trace is displayed as an
upward deflection on the screen.
|
|
|
As the CAP progresses between both recording
electrodes, the recorded
potential returns to the base line (no voltage difference
between the two recording electrodes). |
|
|
As the CAP passes the second electrode (R2), a deflection of the
same size but opposite sign will be recorded. The sign is negative because of the
way the amplifier compares the two inputs. |
|
|
Theoretically, if the electrodes are sufficiently far
apart, a short segment of 0 deflection will be recorded before the
CAP reaches the second electrode. It may not or may not be difficult
to observe this very short segment: the recording electrodes have to be
sufficiently far apart. If in a frog nerve at room temperature, the
duration of the action potential is about 1.5 ms, and the conduction
velocity is about 20 m/s, what would be the
distance occupied by the active region of the action potential? How far
apart should the recording electrodes be in order to see a short segment
of 0 deflection? |
|
|
If the electrodes are not separated by such a large
distance, the two phases will not be of equal amplitude. The CAP will not have
completely passed the first electrode before reaching the second. Adding the two
opposite signed deflections will reduce the amplitude of the negative phase and decrease
the apparent width of both. |
|
|
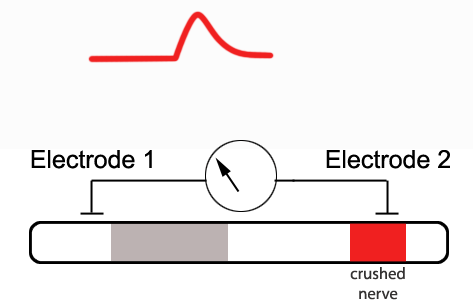
If one were to crush the nerve at the second
electrode -effectively permanently depolarizing the membrane at this
location - a monophasic CAP would result.
|
|
|
Click here to continue with the topic of Experimental
preparation |