|
Biochemical/molecular techniques |
Background concepts:
Immunoprecipitation |
| |
Typically, a blood or urine
sample contains a variety of proteins (crude sample) while only one may
be of diagnostic interest. Immunoprecipitation is often used to
purify a target protein from solution (purified sample). This way, the
protein of interest can be further examined for quantity or physical
characteristics (SDS-PAGE). This technique involves the interaction
between a protein and its specific antibody, the separation of the
immune complexes thus formed with a protein G coated resin or support. |
|
The technique |
Two different approaches are commonly
employed to perform immunoprecipitations.
- An antibody specific to the target
protein is added to the protein mixture. The antigen-antibody complex
is then precipitated from the solution using an insoluble resin which
binds to the antibody complex. Unbound proteins are removed by washing
the resin. The target protein is eluted from the resin for further
analysis. However, the antibody is also collected at this point and
complicates the analysis of the purified target protein.
- a better method is to attach
covalently the antibody to the resin. Then the antibody coupled to
resin is added to a crude protein mixture to capture and precipitate
the antigen (which will become the purified protein). The antigen is
eluted from the resin while the antibody remains covalently bound to
the resin. This simplifies the analysis of the purified protein by not
having the antibody interfere with further analysis such as SDS-PAGE
or protein assays.
|
|
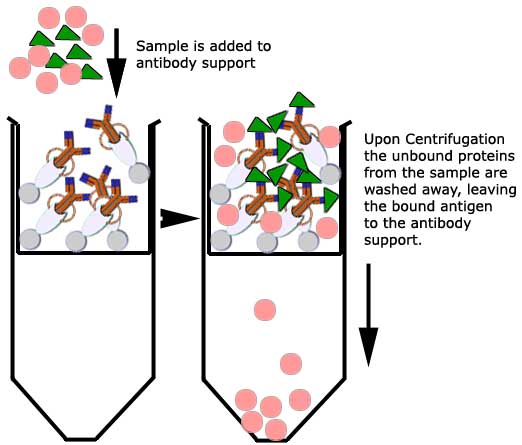
The antibody is bound and
immobilized to a Protein G support using a cross-linking agent
Disuccinimidyl suberate
(DSS). This
properly orients the antibody to "seize" protein (antigen-antibody
complex) from a crude sample applied to the immobilized antibody
support.
Protein G is a bacterial cell wall protein isolated from group G
streptococci: its use in this technique relies on its unique ability to
bind the Fc portion of the antibody and not the Fab fragments of the
antibody. Antigens can thus be isolated by
incubation on a Protein G column that has had their complementary
antibody first bound to the immobilized Protein G. |
|
|
|
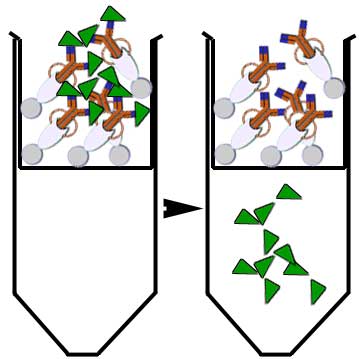
The antigen is then
recovered by elution: a buffer is used to wash and release trapped ions
or molecules from the antibody support (protein G coated beads or
resin).
The immunoprecipitated protein is then collected for further
analysis.
|
 |
|
|