Annie Castonguay.
Background
Annie has obtained her Ph.D. degree (‘08) in organometallic chemistry from the Université de Montréal, under the supervision of Prof. Davit Zargarian and André L. Beauchamp, where she prepared various pincer complexes of nickel and palladium via the activation of aliphatic C-H bonds, and studied their reactivity. On the completion of her thesis, she went to Boston for a postdoctoral position at Tufts University in the group Prof. Elena Rybak-Akimova, in collaboration with the labs of Prof. Christopher Cummins at MIT (‘08-09), where she studied the kinetics of fast reactions involving very sensitive complexes of molybdenum able to activate atmospheric dinitrogen. To further her experience in organic synthesis, she accepted a postdoctoral position at McGill University, where she worked with Prof. Ashok Kakkar and Prof. Theo van de Ven in collaboration with the Finnish company Kemira (‘09-11) on the preparation and the functionalization of macromolecules of interest to industry. She has gained some experience in biology (‘12) when she joined the group of Prof. Dusica Maysinger (McGill’s Faculty of Medicine), and worked on the development of multitasking organometallic complexes for breast cancer therapy. She is currently a postdoctoral fellow in the groups of Prof. Chao-Jun Li, Dusica Maysinger and Audrey Moores (McGill University, Faculty of Medicine/Science) and works on a chemical biology / green chemistry project involving the preparation of nanosensors for biological applications. In a few months, Annie will join the Institut National de la Recherche Scientifique (INRS-IAF) as a professor of chemistry (http://www.inrs.ca/annie-castonguay).
Research highlight
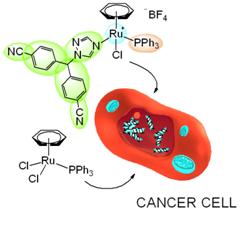
- Multitasking Drugs for Breast Cancer Therapy
Breast cancer is the most frequently diagnosed cancer and the leading cause of cancer death among women, accounting for 23% of total cancer cases in women, and 14% of cancer deaths. An appealing strategy to increase the survival probability of breast cancer patients is to create multitasking drugs, able to promote cell death by simultaneously targeting selected signal transduction molecules. We have recently undertaken the preparation of ruthenium-letrozole multitasking drugs. Ruthenium compounds are well-known for their selective cytotoxicity to cancer cells, whereas letrozole is an aromatase inhibitor which was approved by the FDA as a first-line treatment, due to its proven superiority to the widely used prodrug tamoxifen, and which is commonly administered to menopaused women suffering from an estrogen positive (ER+) breast cancer. A significant in vitro activity was established for one of the ruthenium-letrozole complexes against human breast cancer MCF-7 cells, and interestingly, a much lower activity was noted for the same complex against human glioblastoma U251N cells.
- Development of nanosensors for biology applications
To date, there exists no convenient way to quantify the activity of many enzymes, such as aromatase. Aromatase has an important role in sexual differentiation, fertility, and carcinogenesis. Thus, the development of new sensors to evaluate the activity level of that enzyme is of high importance. The assays developed so far often require the use of radioactive materials and specialized equipment for radiometric measurement since they rely on measuring the amount of tritiated water released upon the conversion of a radiolabeled androstenedione. We recently undertook the preparation of new nonradioactive nanosensors which will allow for the direct quantification of the aromatase activity by ratiometric fluorescence detection. We are using “click” chemistry for the preparation of the sensors, and are exploiting the fluorescence resonance energy transfer (FRET) between fluorophores and gold nanoparticles to measure the enzyme’s activity.
Fellowships & awards
- CIHR - Travel Award - “Metals in Biology” Gordon Research Conference (Jan.14) - $1,000
- CIHR – Travel Award - “New PI Meeting” - Canadian Cancer Research Conference (Nov. 13) - $1,500
- CIHR - Chemical Biology Postdoctoral Scholarship (McGill, Jan. 13) - $18,000
- Faculté des Études Supérieures Scholarship (UdeM, May 03-04), for direct entry to the Ph.D. program - $14,000
- Charron-Lam Scholarship (UdeM, Oct. 05) - $5,000
- Merck / Marc Labelle Travel Award (UdeM, Sept. 06) - $1,000
- Best Poster Award at the 36th Inorganic Discussion Weekend, McMaster University (Nov. 03)
Publications
- “Click” Reactions: an Emerging Tool for Biology, Frontiers of Nanobiomedical Research.
Castonguay A, Maysinger D.
(invited book chapter edited by Vladimir P. Torchilin, submitted).
- ECE-Type Pincer Complexes of Nickel, Topics in Organometallic Chemistry 2013, 40, 131
Zargarian D, Castonguay A, Spasyuk DM.
(invited book chapter from “Organometallic Pincer Chemistry”, edited by Gerard van Koten and David Milstein).
- New Ruthenium(II)-Letrozole Complexes as Anticancer Therapeutics.
Castonguay A, Doucet C, Juhas M, Maysinger D.
J Med Chem. 2012 Sep 19. [Epub ahead of print]
- Dendrimers as Bactericides.
Castonguay, A.; Ladd, E.; van de Ven, T. G. M.; Kakkar, A.
New Journal of Chemistry 2012, 36, 199.
- Thermosensitive dendrimer formulation for drug delivery at physiologically relevant temperatures.
Castonguay A, Wilson E, Al-Hajaj N, Petitjean L, Paoletti J, Maysinger D, Kakkar A.
Chem Commun (Camb). 2011 Nov 28;47(44):12146-8.
- Dendrimer templated construction of silver nanoparticles.
Castonguay A, Kakkar AK.
Adv Colloid Interface Sci. 2010 Oct 15;160(1-2):76-87.
- Combined Cu(I)-catalysed alkyne-azide cycloaddition and furan-maleimide Diels-Alder "click" chemistry approach to thermoresponsive dendrimers.
Vieyres A, Lam T, Gillet R, Franc G, Castonguay A, Kakkar A.
Chem Commun (Camb). 2010 Mar 21;46(11):1875-7.
- Coordination-mode control of bound nitrile radical complex reactivity: intercepting end-on nitrile-Mo(III) radicals at low temperature.
Germain ME, Temprado M, Castonguay A, Kryatova OP, Rybak-Akimova EV, Curley JJ, Mendiratta A, Tsai YC, Cummins CC, Prabhakar R, McDonough JE, Hoff CD.
J Am Chem Soc. 2009 Oct 28;131(42):15412-23.
- Regioselective Hydroamination of Acrylonitrile Catalyzed by Cationic Pincer Complexes of Nickel.
Castonguay, A.; Spasyuk, D. M.; Madern, N.; Beauchamp, A. L.; Zargarian, D.
Organometallics 2009, 28, 2134.
- New Derivatives of PCP-Type Pincer Complexes of Nickel.
Castonguay, A.; Beauchamp, A. L.; Zargarian, D.
Inorganic Chemistry 2009, 48, 3177.
- Preparation and Reactivities of PCP-Type Pincer Complexes of Nickel. Impact of Different Ligand Skeletons and Phosphine Substituents.
Castonguay, A.; Zargarian, D.; Beauchamp, A. L.
Organometallics 2008, 27, 5723.
- Ni(II) complexes featuring non-metallated pincer-type ligands.
Pandarus V, Castonguay A, Zargarian D.
Dalton Trans. 2008 Sep 21;(35):4756-61.
- Trans,trans-Bis[µ-1,5-bis(diisopropylphosphino)pentane-κ2P :P']bis[dichloronickel(II)].
Castonguay, A.; Beauchamp, A. L.; Zargarian, D.
Acta. Cryst. 2007, E62, m196.
- Catalytic Reactivities of Indenyl-Nickel, Indenyl-Palladium, and PCsp3P-Nickel Complexes.
Sui-Seng, C.; Castonguay, A.; Chen, Y.; Gareau, D.; Groux, L. F.; Zargarian, D.
Topics in Catalysis 2006, 37, 81.
- Syntheses and Reactivities of New Pincer Complexes of Nickel.
Castonguay, A.; Sui-Seng, C.; Zargarian, D.; Beauchamp, A. L.
Organometallics 2006, 25, 602.
- Chloro[η5/-1-(dimethylaminoethyl)indenyl]- (triphenylphosphine)nickel(II) diethyl ether hemisolvate. Castonguay A.; Beauchamp, A. L.; Vachon, J.; Zargarian, D.
Acta. Cryst. 2005, E61, m1512.
- {2,6-Bis[(dimethylamino)methyl]phenyl-κ²N,C1,N'}-chloronickel(II).
Castonguay, A.; Charbonneau, F.; Beauchamp. A. L.; Zargarian, D.
Acta. Cryst. 2005, E61, m2240.